Agricultural plastic film | Environmentally friendly degradable film | Self adhesive protective film
Conductive film | High barrier cling film | Heat shrink film
Welcome to Changzhou Hefeng Packaging Materials Co., Ltd!
Agricultural plastic film | Environmentally friendly degradable film | Self adhesive protective film
Conductive film | High barrier cling film | Heat shrink film
Abstract
In this study, an economical and efficient sol gel method was proposed to prepare nano porous silicon dioxide (SiO ₂) films with both high transmittance and super hydrophobicity to improve the efficiency of solar cells. By adding trimethylethoxysilane (TMES) as a hydrophobic modifier to the alkaline catalyzed mixed sol, the optimized SiO ₂ film achieved a peak transmittance of 97.05% (5.56% higher than bare glass) and a water contact angle of 150 °, demonstrating excellent anti reflection and self-cleaning properties. A systematic analysis of the nanoporous structure was conducted using scanning electron microscopy (SEM) and theoretical models, with a characteristic critical particle gap (Dc) of 40 ± 10 nanometers. The results showed that air entrapment caused by surface roughness significantly contributed to hydrophobicity. Spectral response simulation shows that the increase in transmittance is directly proportional to the increase in short-circuit current (Δ Jsc), confirming the potential of this thin film to improve the efficiency of solar cells. Durability testing has confirmed that its performance has only minimal degradation (after 5000 water droplet impacts, transmittance loss of 0.31% and contact angle reduction of 15 °), highlighting its strong environmental adaptability. This work not only provides a scalable approach for multifunctional anti reflective coatings, but also links material design with photovoltaic applications, providing insights for the development of sustainable energy technologies
introduction
To improve the efficiency of solar cells, increasing the transmittance of surface incident light is a very effective method. In the process of absorbing solar photons and generating photoelectric effects, there are two factors that can reduce the photoelectric conversion efficiency of solar cells. Firstly, there is electrical loss, which occurs when the material cannot absorb photons with energy below the frequency band gap; Any excess energy of photons with energy higher than the frequency band gap is considered as heat loss. Secondly, there is optical loss, which is caused by the high refractive index of the material. This results in a portion of the incident photons being reflected and lost before being absorbed. According to the principle of Fresnel reflection, coating one or more layers of anti reflective coatings on the surface of solar cells can help reduce the reflection of incident light, thereby increasing transmittance and improving the effective absorption of light tons. If glass is used as the substrate (ns=1.52), the ideal refractive index of a single-layer anti reflection film should be 1.23. However, the traditional dense matrix that closely matches this ideal refractive index is magnesium fluoride (n=1.38), which exceeds the ideal value. In order to solve this problem, the development of porous films is rapid, and a large number of studies have been carried out to prepare porous films by sol-gel method. Wong et al. prepared highly dispersed SiO2 sol in an alkaline catalytic system, achieving a transmittance of 99.307% at 266nm and a surface roughness of 1.339nm. The film exhibited excellent stability in terms of transmittance. Xu et al. pointed out that porosity is inversely proportional to refractive index. Due to the high porosity of these materials, low refractive index porous films can be achieved. This article investigates the preparation of high transmittance SiO2 thin films using an alkaline one-step method.
Add organic surface modifiers in an alkaline catalytic environment to form a mixed sol doped with TMES. SiO2 thin films with superhydrophobicity and high transmittance were prepared by immersion drawing method. We conducted infrared absorption spectroscopy replication, scanning electron microscopy, water contact angle measurement, and durability testing on the film. Through analysis, a theoretical model for the surface roughness of thin films has been proposed, providing a reasonable explanation for the high transmittance and strong hydrophobicity of thin films
As is well known, anti reflective coatings on the surface of solar cells can increase current output by several percentage points, but poor hydrophobicity in outdoor environments can hinder the performance of the coating. Therefore, the coating with high transmittance and super hydrophobicity was prepared by low-cost sol gel impregnation method. This article guides the development of high transmittance superhydrophobic nanoporous SiO2 thin films through experimental methods. We conducted an in-depth analysis of the spectral response theory related to solar cells using the photovoltaic current model, and examined the influence of nano anti reflective films on their spectral response. We simulate the spectral response of solar cells by calculating the weighted average transmittance of thin films and evaluate how an increase in transmittance leads to an increase in short-circuit current. Our goal is to enhance the effective transmission of photons, thereby improving the anti reflection properties and spectral response of solar cell anti reflection films. This improvement is crucial for increasing the photocurrent of the battery, which is of great significance for improving the overall battery efficiency. The relevant literature systematically explores the influence of anti reflection on short-circuit current. The surface of the film has anti reflection properties. Compared with bare silicon wafers, silicon wafers with anti reflection films can achieve a significant increase in maximum current by 76.9%.
Experimental Section
Preparation of Sol
The hybrid sol doped trimethylethoxysilane (TMES) was prepared by one-step sol gel alkali method, and the hybrid sol was named SolC. Before conducting the experiment, all equipment, including beakers, measuring cylinders, and droppers, were cleaned with acid and then subjected to ultrasonic cleaning for 30 minutes. Finally, rinse the equipment with deionized water and dry it in an oven for future use. Preparation steps:
Step 1: Add 1.3mL of ammonia water to 40mL of anhydrous ethanol, and stir the resulting mixture magnetically at a constant temperature of 50 ℃ for 30 minutes.
In the second step, mix 1mL of ethyl orthosilicate with 0.8mL of TMES, and then add the mixture dropwise to the solution prepared in the first step to form the final reaction solution.
In the third step, the final reaction solution is stirred magnetically at 35 ° C for 24 hours, and then aged at room temperature (28 ° C) for 6 days (or at least 4-6 days to achieve a uniform sol), thereby forming a mixed sol C. This sol can usually be stored for about a month; However, over time, the size of the sol particles gradually increases.
Preparation of thin films
This article uses the immersion pulling method to immerse the processed glass slide in sol C for about 5 seconds. Then, the glass slide is quickly pulled out at a constant speed of 3mm/s, consistent with the pulling method previously used in this work. Allow the coated substrate to naturally dry at room temperature (approximately 28 ℃) to form a thin film designated as C1 without any heat treatment. The dried film C1 is then placed in a 200 ℃ oven for 30 minutes to produce a second film, called C2. We will also conduct droplet testing in the future and label the tested film as C3 (Figure 1).
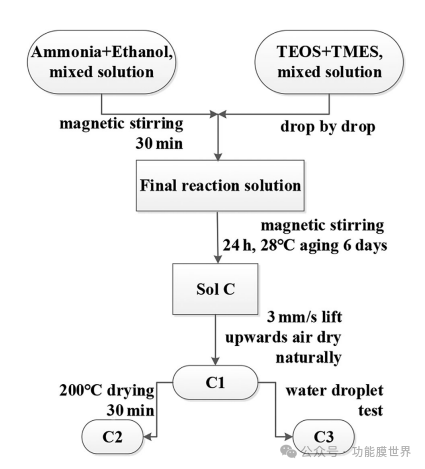
Figure 1 Preparation process of sol C and thin films C1, C2, and C3.
Sample testing
Infrared Fourier Transform Spectrophotometer, model FTIR-8400 (Shimadzu, Japan), is used to analyze specific chemical bonds and functional groups within thin films. Use a UV-3600 (Shimadzu, Japan) UV-VIS-NIR spectrophotometer for transmittance testing, covering a wavelength range of 380-900nm. Use a contact angle measuring instrument produced by Shanghai Zhongchen Digital Technology Equipment Co., Ltd., model JC2000DS2, to measure the water contact angle (WCA) to evaluate the hydrophobicity of the thin film. During the measurement process, 5 μ L water droplets were applied and the droplet images were captured using a CCD camera at a speed of 50 frames per second. Use scanning electron microscopy models S-4800 (Hitachi) and XL30 (Philips) to examine the microstructure and surface morphology of the thin film.
Results and Discussion
Research on the Doping Mechanism of TMES
Theoretical simulation of rough surfaces: When the contact angle exceeds 90 degrees, the wettability of the film decreases with increasing surface roughness. This indicates that under hydrophobic conditions, the larger surface roughness of the membrane enhances its hydrophobicity. According to research on the surface structure of nanopores, air capture is also a key factor affecting wettability. The particle gap (Dc) is a prerequisite for determining the presence of trapped gases, as shown in the following equation:
image
In the formula, γ represents surface tension, n1 represents the molecular density of the droplet, and j μ 1 μ gj represents the absolute difference in chemical potential between the liquid phase and the gas phase. Under normal temperature and pressure conditions, the critical value (maximum value) of Dc is 100nm. When the gap Dc between two particles is less than 100nm, a large number of bubbles will form between the particles, causing water on the particles to be replaced by the bubbles. In other words, the surface structure and porosity of the film trap gases in these gaps, thereby achieving a hydrophobic effect.
Analysis of Particle Growth Mechanism: TMES is a commonly used hydrophobic material. As a trifunctional organosilane, this work combined it with ethyl orthosilicate as a co precursor to synthesize silica sol containing hydrophobic groups. When ammonia is used as a catalyst, the hydrolysis rate of ethyl silicate is higher than its solidification rate, resulting in the formation of granular microspheres. Most of the hydrophilic hydroxyl groups (OH) initially present on the surface are replaced by hydrophobic methyl groups (CH), which is consistent with the previous research results discussed in this work. [8] The schematic diagram is shown in Figure 2.
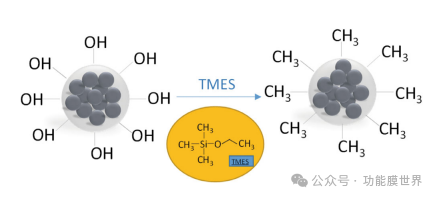
Figure 2: Growth model of modified particles, illustrated as the molecular formula of trimethylethoxysilane (TMES).
Spectral response analysis of solar cell efficiency
Theoretical simulation of solar cell efficiency: The definition formula of solar cell conversion efficiency η is as follows:
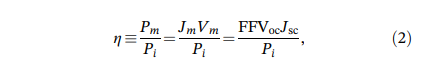
In the formula, Pm represents the maximum output power per unit area of the solar cell, while Jm and Vm represent the maximum current and voltage at that moment. Pi is the unit area of solar power projected onto the surface of the battery. FF, Voc, and Jsc refer to the fill factor, open circuit voltage, and short-circuit current density of solar cells, respectively. From this equation, it can be inferred that to determine the conversion efficiency η, research must focus on the fill factor FF, open circuit voltage Voc, and short-circuit current density Jsc. In order to measure the efficiency of solar cells, it is necessary to coat the surface of the cell. By coating the surface of the solar cell and measuring the corresponding parameters before and after coating, such as FF, Voc, and Jsc, the improvement in cell efficiency can be evaluated. Due to the limitations of depth and duration of this study, this article only conducts relevant simulation calculations on the increased short-circuit current Jsc, indirectly reflecting the improvement of solar cell efficiency.
Short circuit current calculation: This article calculates the weighted average transmittance and increment in short-circuit current simulation after preparing thin film coating. When determining the weighted average transmittance in the visible light range of 400-800nm, the increment of short-circuit current is calculated using theoretical formula, denoted as Δ Jsc. The theoretical calculation formula is as follows:
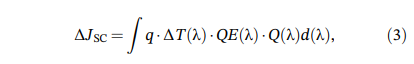
Where q is the amount of charge, q=1.61019(As); Δ T (λ) is the increased transmission spectrum, Δ T λð 222¼ TC1 λ Tray glass λð 222; QE (λ) is the quantum efficiency, assumed to be 100% here; And Q is the photon density flow of the solar spectrum. Taking the AM1.5 solar spectrum, it can be seen that the short-circuit current increment Δ Jsc is a value related to the transmittance increment, but this calculation is still completely ideal
The calculation of transformation.
Analysis of Film Composition
The chemical bonds and specific functional groups (CH3) in C1, C2, and C3 thin films were determined using infrared absorption spectroscopy, as shown in Figure 3. The curves of the films almost overlap, indicating that the composition of the three films is basically the same, with very little difference. The absorption peak near 3400cm-1 corresponds to the antisymmetric stretching vibration of OH groups, indicating that the surface of the mixed sol coating prepared by alkali catalysis contains a higher concentration of OH groups. In addition, the curve exhibits clear absorption peaks at 2900 and 2850 cm-1, attributed to the carbon hydrogen bonds in methyl (CH3), confirming the presence of a large number of methyl groups on the surface of the film. The vibration peak near 1615cm-1 is related to the stretching vibration of carbon and oxygen, indicating the presence of residual organic compounds or esters in the film. All curves also exhibit strong vibration peaks around 900cm-1, which are related to the stretching (bending) vibration of silicon oxygen silicon bonds. All results indicate that the main component of the coating is SiO2, and the coating surface contains abundant CH3. The CH3 groups observed on C1, C2, and C3 are attributed to the addition of TMES. Therefore, after coating, a large amount of methyl groups are introduced into the surface of the film, resulting in a superhydrophobic coating.
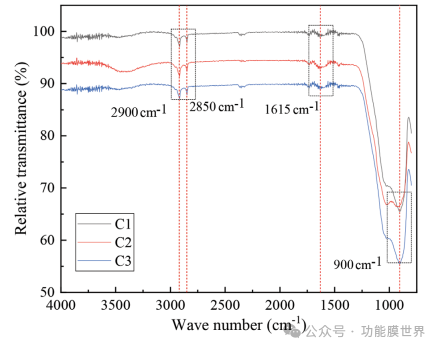
Figure 3: FTIR spectra and infrared Fourier transform of samples C1, C2, and C3.
Optical Performance Analysis
The transmittance curves of thin films C1, C2 and bare glass are shown in Figure 4. From the figure, it can be seen that the peak transmittance of the thin film C1 prepared by TMES doped mixed sol at 472nm reaches 97.05%. This is 5.56% higher than the peak transmittance of bare glass, which is 91.49%. The peak transmittance of film C2 appears at 474nm, with a value of 96.73%, which is 5.24% higher than that of bare glass. The curve and peak values indicate that the film has excellent anti reflection performance, mainly reflecting the high transmission performance of the alkali catalyzed sol, reaching a high standard. It was also observed that after treatment at 200 ℃, the peak transmittance and overall transmittance curve of film C1 slightly decreased. This decrease may be attributed to the evaporation of organic components in the wet film during heat treatment, resulting in a dense film and a decrease in porosity. By analyzing the trend of the curve and comparing the peak values before and after heat treatment, we can conclude that the effect of heat treatment on the transmittance of the film can be ignored.
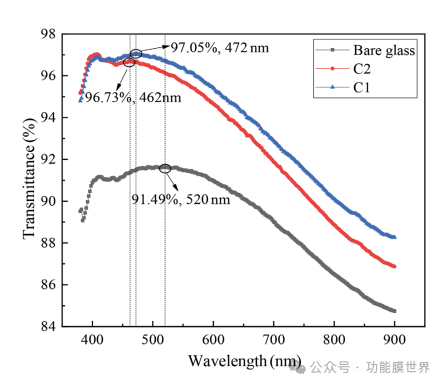
Figure 4: Measurement of transmission spectra of thin films C1, C2 and bare glass.
Hydrophobic performance analysis
Hydrophobic treatment significantly improves the contact angle of the film. The main reason for the increase in hydrophobicity is the presence of a large number of hydrophobic methyl groups (CH3) in the surface modifier, which can displace a considerable amount of hydrophilic hydroxyl groups (OH) on the membrane surface. The superhydrophobic membrane described in this article is based on the principle of hydrophobic groups replacing hydrophilic groups. In the initial preparation process of the sol, TMES containing high concentrations of methyl groups is mixed with ethyl orthosilicate as a co precursor to produce a mixed sol. This process results in the required hydrophobic film while maintaining high transmittance. The hydrophobicity of membrane C1 was evaluated by measuring its water contact angle, and the results are shown in Figure 5.
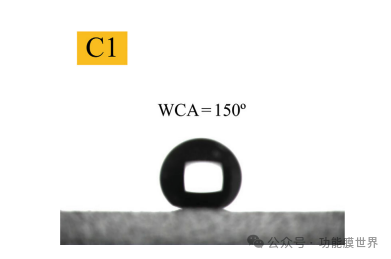
Figure 5: Water contact angle C1 of the film
Thin film C1 is an anti reflective film with excellent performance advantages, cost-effectiveness, and simple preparation process.
Microstructure analysis of thin films
Figures 6A-D show the top plane scanning electron microscopy copy images of thin film C1 at magnifications of 100nm, 200nm, 500nm, and 1 μ m. This series of images shows that the surface of the film is sparse and porous, indicating that the film prepared in this study has a porous structure. This structure explains the low refractive index and high transmittance characteristics of the porous film mentioned earlier. According to relevant theoretical knowledge about hydrophobicity, the roughness of the structure is a key factor in endowing thin films with hydrophobicity. Dc=40 ± 10nm (≤ 100nm). Furthermore, from a series of scanning electron microscope (SEM) images, it is evident that the single particle gap (Dc) can be explained as the distance between two hemispherical points, as indicated by the red arrow in Figure 6A, with Dc=40 ± 10nm (≤ 100nm). In addition, this series of SEM images shows that Dc can be regarded as the distance between two hemispherical points, as indicated by the red arrow in Figure 6A. According to theoretical principles, if the Dc between rough surfaces is less than 100nm, a large number of bubbles will form between the two surfaces. Therefore, it can be inferred that a large number of bubbles are trapped on the rough surface of film C1, significantly improving the hydrophobicity of the film.
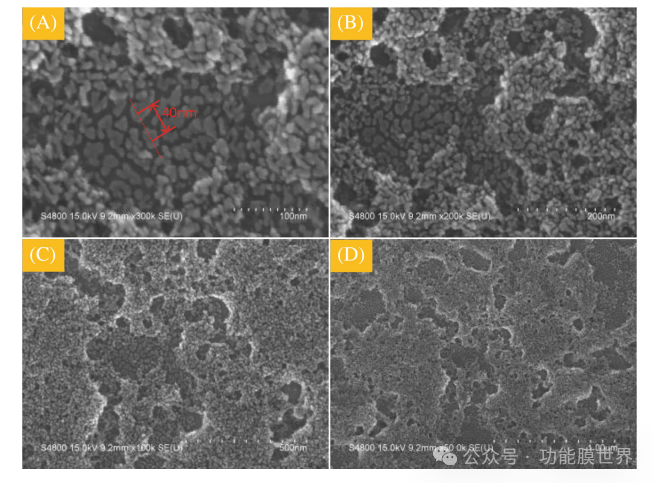
Figure 6: Scanning electron microscopy (SEM) image of the surface morphology of film C1.
durability analysis
The durability of anti reflective films is also a key factor in characterizing their mechanical properties. The wear resistance and adhesion of films prepared by alkaline catalysis are inferior to those obtained by acid catalysis. [17] The thin film produced in this experiment was prepared in an alkaline environment. Due to its excellent hydrophobicity, the water droplet test method was used to evaluate the weather resistance of the film in humid environments. Figure 7 shows a schematic diagram of the machine's testing.
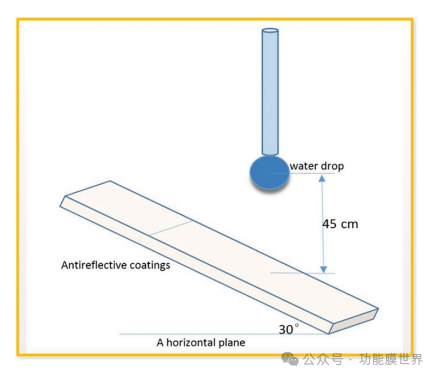
Figure 7: Simulation diagram of water droplet test method.
This article evaluates film C1 using the methodology and program described by Xu et al. A series of 22 μ L water droplets were continuously impacted onto the film surface at a speed of 3m/s for 5000 cycles. After completing the water droplet test, designate the film as C3 and measure the transmittance and WCA value of C3. The results are shown in Figure 8. It can be clearly seen from the comparison chart that the transmittance and water contact angle values of the film have both decreased after testing; However, the changes were relatively small, with a peak transmittance decrease of only 0.31% and a WCA decrease of only 15. This indicates that water droplets have little impact on the environment, which can be attributed to the high transparency and hydrophobicity of the film, making it resistant to moisture and dampness. These characteristics reflect its excellent durability and highlight its significant value for practical applications.
Analysis of Spectral Response Results
The calculation results are shown in the table below. From these results, it can be seen that the peak transmittance and weighted average transmittance of film C1 are significantly higher than those of bare glass. Theoretical calculations show that the short-circuit current increases correspondingly, indicating that the anti reflection film produced in this experiment has effective anti reflection ability. The peak transmittance obtained through testing and the calculated weighted average transmittance are significantly higher than those of uncoated glass plates.
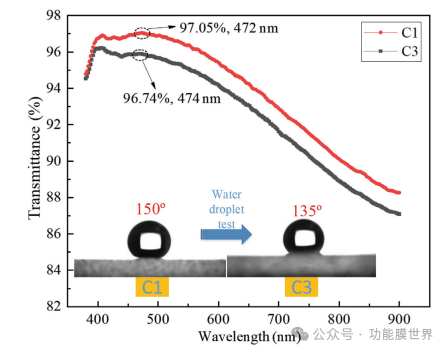
Figure 8: Comparison of Transmittance and Contact Angle after Water Drop Test
Table 1: Maximum transmittance, weighted average transmittance, and ideal short-circuit current increment.
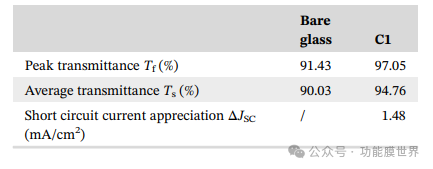
When compared to the ideal increase in short-circuit current, a positive value of Δ Jsc is observed, indicating that the addition of anti reflective film theoretically enhances the short-circuit current of solar cells, which in turn suggests a potential increase in battery efficiency. In addition, the increase in short-circuit current is to some extent proportional to the transmittance of the thin film; The better the transmission performance, the greater the corresponding increase in short-circuit current. This article aims to propose a method to further enhance the photocurrent of solar cells by increasing the effective transmittance of photons, thereby improving the photoelectric conversion efficiency of solar cells (Table 1).
Conclusion
In this paper, TMES doped hybrid sol was prepared by sol-gel method, and superhydrophobic SiO2 antireflection coating with high transmittance was obtained by dipping and drawing on glass substrate. The coating presents a nano porous structure with a maximum transmittance of 97.05%. Infrared spectroscopy testing shows that there are a large number of methyl groups on the surface of the film. The addition of TMES indicates that the hybrid sol contains a large amount of methyl groups, resulting in the formation of a hydrophobic thin film with a water contact angle of up to 150.
Scanning electron microscopy analysis shows that the surface of the film has a nanoscale rough porous structure, providing a solid foundation for theoretical analysis of the transmission and hydrophobic properties of the film. By simulating graphics and utilizing theoretical knowledge, we elucidated the influence of rough surfaces on transmission and wetting properties. The durability test of the film showed that the transmittance and water contact angle only slightly decreased, proving the good durability of the film. In addition, simulations and calculations have confirmed that the addition of anti reflective films improves the efficiency of solar cells by increasing short-circuit current. Theoretical analysis and simulation calculations confirm that anti reflective coatings significantly improve the efficiency of solar cells and contribute to energy conservation.
Through analysis and simulation studies on transmittance, film surface structure, water contact angle, durability, and other properties, we have verified the superior performance of anti reflection films in practical applications. These results provide new theoretical and practical significance for the field of anti reflective films. In this study, it is concluded that a simple and efficient sol-gel method can successfully prepare SiO2 antireflection coatings with high transmittance and superhydrophobicity.
Welcome to Changzhou Hefeng Packaging Materials Co., Ltd!
Mobile phone: 15895061333
Email: 115555372@qq.com
Address: Moujia Village, Zhenglu Town, Tianning District, Changzhou City
Copyright © 2025 Changzhou Hefeng Packaging Materials Co., Ltd