Agricultural plastic film | Environmentally friendly degradable film | Self adhesive protective film
Conductive film | High barrier cling film | Heat shrink film
Welcome to Changzhou Hefeng Packaging Materials Co., Ltd!
Agricultural plastic film | Environmentally friendly degradable film | Self adhesive protective film
Conductive film | High barrier cling film | Heat shrink film
Abstract
Due to its photocatalytic self-cleaning properties, titanium dioxide modified anti reflective coatings (ARCs) on photovoltaic glass have become a research hotspot. However, balancing the optical performance, photocatalytic performance, weather resistance, and process complexity of composite nano coatings remains a challenge. In this study, rutile type nano TiO ₂ with an initial particle size of 5 nanometers was embedded into the surface of closed cell anti reflection coatings (CPARCs) using a layer by layer coating method, and a multifunctional anti reflection coating that perfectly solves the above problems was prepared. The scanning electron microscope (SEM) results indicate that the nano TiO ₂ - modified CPARCs have an ideal closed pore structure, and the nano TiO ₂ particles are well dispersed on the surface of CPARCs. Fourier transform infrared spectroscopy (FT-IR) and deep etching X-ray photoelectron spectroscopy (XPS) confirmed that during the calcination process, a Si-O-Ti chemical bond was formed between the nano TiO ₂ and the silicon dioxide on the coating surface, indicating a strong connection between the two. TiO ₂ - modified bilayer CPARCs can effectively balance the light absorption and reflection caused by TiO ₂, and their optical properties are even better than CPARCs. Compared with ordinary glass, the average transmittance in the wavelength range of 400-700 nanometers and 400-1100 nanometers is 97.53% and 95.86%, respectively, and the gain effect is 6.76% and 6.05%, respectively. In addition, the photocatalytic performance of the coating has been proven to be superior to coatings that directly incorporate nano TiO ₂ into the system. In addition, atomic force microscopy (AFM) observation showed that after TiO ₂ was embedded on the surface of the coating, the roughness of the coating increased, and the contact angle decreased from 24.2 ° to 6.2 °. After UV irradiation, it could be reduced to 3.6 °, achieving a superhydrophilic effect. CPARCs modified with TiO ₂ also exhibit excellent wear resistance and weather resistance. This is a new, simple, and efficient preparation method.
introduction
As a renewable energy technology that converts sunlight into electricity, photovoltaic (PV) power generation has received much attention in recent years in response to severe energy crises. In practical applications, it has been found that the power generation efficiency of photovoltaic modules decreases over time. One of the reasons for this result is that the surface of the photovoltaic panel is covered with dust and other impurities. Research has shown that photovoltaic modules with a surface dust layer of 4 grams per square meter may reduce their power generation efficiency by 40%. Therefore, it is necessary to regularly clean the photovoltaic modules.
However, cleaning photovoltaic modules has always been a challenge. At present, the main cleaning methods include rainwater cleaning, robot cleaning, and mechanical cleaning. These methods have the disadvantages of strong environmental dependence, low cleaning efficiency, and high cost. Another possible way to solve the cleaning problem of photovoltaic modules is to use anti reflective coatings (ARCs) with self-cleaning function. Compared with the above methods, anti reflective coatings with self-cleaning function are more efficient and convenient. Therefore, self-cleaning anti reflective coatings have become an important research hotspot, which is expected to solve the problems of light reflection and dust pollution on the surface of photovoltaic glass.
The principle of self-cleaning coating is mainly based on the photocatalytic performance and wettability of the surface [8]. Due to its excellent photocatalytic performance, stability, and non-toxic chemical properties, TiO ₂ is currently the most widely used photocatalytic material. However, compared to SiO ₂, TiO ₂ itself has a higher refractive index, making it difficult to prepare anti reflective coatings alone. Therefore, since the Fujishima research group first introduced TiO ₂ into anti reflective coatings, TiO ₂ modified SiO ₂ anti reflective coatings have become the focus of research. There are three main strategies for preparing TiO ₂ - modified SiO ₂ anti reflective coatings.
The first conventional method is to construct a SiO ₂&TiO ₂ double shell structure of nanospheres to form an anti reflective coating. For example, Yao et al. synthesized hollow nanospheres with SiO ₂ and TiO ₂ double shells by continuous sol-gel method to design and optimize antireflection coatings. However, the dense TiO ₂ shell in this system can reduce the optical performance of the coating, and more and more researchers are exploring anti reflective coatings based on raspberry shaped SiO ₂ @ TiO ₂ composite nanospheres. For example, our group designed nano TiO ₂ - modified polyacrylate/silica core-shell nanoparticles through electrostatic self-assembly, and obtained a self-cleaning functional anti reflective coating through immersion coating and heat treatment processes. Wu et al. synthesized uniform raspberry shaped hollow SiO ₂ @ TiO ₂ composite nanospheres and prepared anti reflective coatings. Its transmittance gain effect in the visible light range is over 7%, demonstrating the advantage of its raspberry like structure. The second commonly used method is to directly mix TiO ₂ nanoparticles with silica nanoparticles or hollow silica nanospheres to form an anti reflective coating. For example, Li et al. prepared a mixed dispersion of silica nanoparticles, commercial Degussa P25 titanium dioxide nanoparticles, and acid catalyzed silica sol, and prepared a titanium dioxide modified silica anti reflective coating with mechanical strength, enhanced light transmittance, and photocatalytic self-cleaning performance through a dip coating process. The third common method for preparing titanium dioxide modified silica anti reflective coatings is layer by layer deposition. Fujishima Akira's research team used this method to obtain a multifunctional anti reflective coating on the surface of a silica coating on glass by layer self-assembly of electrons using self-made titanium dioxide sol or nanosheets. However, the results indicate that even ultra-thin nano titanium dioxide layers can still lead to a decrease in optical performance, and mechanical properties have not yet been validated. Subsequently, Fostini prepared a double-layer titanium dioxide/silicon dioxide anti reflection coating and enhanced the optical properties of the system by reducing the refractive index of the top titanium dioxide layer. This was achieved by using PB-b-PEO block copolymer as a pore forming agent to form uniformly ordered nanopores. Later, Jin et al. designed a well structured macroporous mesoporous bilayer silica/titanium dioxide anti reflection coating and demonstrated that this nanostructured film still exhibits broadband anti reflection performance, far superior to traditional planar silica/titanium dioxide films.
In fact, as of now, TiO ₂ - modified SiO ₂ anti reflective coatings have not been applied in the photovoltaic industry. The main reasons are as follows: (1) It is difficult to balance the anti reflection performance and photocatalytic performance of the system. (2) The hydrolysis and condensation reactions of TiO ₂ precursor are very fast, and the process conditions are difficult to control. (3) The introduction of TiO ₂ causes a change in the zeta potential of the system, reducing the storage stability of the dispersion.
Introducing commercial nano titanium dioxide into silica anti reflective coatings can make the preparation process more efficient, economical, and beneficial for practical applications. As a star product, P25 with a particle size of about 25 nanometers is often introduced into functional photocatalytic coatings. However, P25 has a larger particle size, which can cause strong light scattering and damage the anti reflection performance of the coating. In addition, as shown in Figure S3, P25 cannot be stably dispersed in water and will completely precipitate within 1 hour. In addition, due to the high refractive index of titanium dioxide, its usage in silica anti reflective coatings is limited. To further improve photocatalytic performance, titanium dioxide with stronger photocatalytic ability than P25 should be preferred.
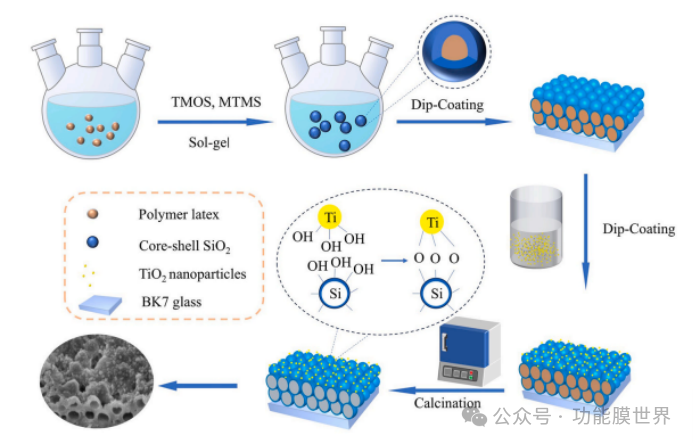
图1.碳包覆多孔无定形锐钛矿(CPARCs)和Tiₓ-碳包覆多孔无定形锐钛矿(Tiₓ-CPARCs)制备过程的示意图。
Inspired by the above research, this article adopts a multi-layer deposition method to introduce a preferred commercial nano TiO ₂ onto the surface of closed cell structure anti reflective coatings (CPARCs). The nano TiO ₂ particle size is only a few nanometers, and its photocatalytic ability is 2.3 times that of P25. The preparation process of CPARCs is shown in Figure 1. The nano titanium dioxide on the coating surface is designed to be discontinuously distributed to reduce the influence of high refractive index. The nano TiO ₂ modified SiO ₂ anti reflective coating prepared by this method not only has excellent anti reflective and photocatalytic properties, but also greatly reduces the amount of TiO ₂ used, which is beneficial for the practical application of this technology. It is worth mentioning that the medium for both closed cell SiO ₂ dispersion and TiO ₂ dispersion is water, making it an environmentally friendly coating system.
Experimental Section
material
Tetraethoxysilane (TEOS, ≥ 99%) was purchased from SigmaAldrich; Hydrochloric acid (HCl, 37%), methylene blue (MB, 98%), ethanol (EtOH, ≥ 99.7%), and BK7 glass (Sel brand, size: 7.5cm × 2.5cm × 0.1cm) were purchased from Shanghai Titan Technology Co., Ltd; Styrene (St, ≥ 99.8%), methyl acrylic acid (MAA, ≥ 99.8%), ammonium persulfate (APS, ≥ 99.8%), and sodium dodecyl sulfonate (SDS, ≥ 99.8%) were purchased from China National Pharmaceutical Group Chemical Reagent Co., Ltd; Tetramethoxysilane (TMOS, ≥ 98%) and methyltrimethoxysilane (MTMS) were purchased from Jianghan Fine Chemical Co., Ltd. Nano TiO ₂ PT05 and JWN-TO-A01 are provided by Nanjing Baokete New Materials Co., Ltd. and Ningbo Jiwei Nano New Materials Technology Co., Ltd. respectively. The experimental water used is laboratory made deionized water.
Synthesis of dispersion of core-shell polystyrene/silica composite particles
Firstly, referring to the literature, a latex template was synthesized and slightly modified by replacing methyl methacrylate and butyl acrylate in the formula with styrene to obtain a more suitable particle size. Afterwards, dilute 50g of latex template with deionized water to 500g, place it in a flask, stir at 350rpm, and adjust the temperature to 35 ℃. Subsequently, 6.67gTMS was added dropwise into the flask at a constant rate of 20mL/h using a multifunctional injection pump. Continue stirring for 30 minutes after the dropwise addition is complete. Next, 43.33gTMS was added in the same manner. After the dropwise addition is completed, continue to react for 36 hours and age for 2 days for later use.
Preparation of Acid catalyzed Silica Sol (ACSS)
The preparation of ACSS has been slightly modified according to the reference literature. Firstly, mix 67.5mL of ethanol with 2.25g of deionized water to form a homogeneous solution. Then add 0.125g of concentrated hydrochloric acid dropwise to adjust the pH value. Afterwards, add 7mLTEOS at a certain rate. Finally, stir the solution in a sealed glass container for 4 hours and let it age at room temperature for at least 4 days before setting it aside. ACSS is used as the bottom layer of double-layer anti reflective coatings, with a high refractive index.
Preparation of CPARCs and TiO ₂ - modified CPARCs
The glass substrate is sequentially cleaned with glass cleaner, deionized water, and ethanol to ensure surface cleanliness. All coatings were prepared by immersion method using SYDC-200 immersion and pulling machine at 25 ℃, and the coating thickness was controlled by adjusting the pulling rate. Mix a certain amount of nano TiO ₂ powder with deionized water and stir magnetically to form a 1mg/mL transparent sol. CPARCs remove organic components by impregnating and coating a dispersion of core-shell polystyrene/silica composite particles onto the surface of BK-7 glass, and calcining at 550 ℃ for 10 minutes. The preparation of Ti ₓ - CPARCs (x is the pulling rate, 500-2500 μ m/s) is as follows: the first layer of the coating is impregnated with a core-shell composite particle dispersion and dried at room temperature for 5 minutes; Subsequently, TiO ₂ sol was impregnated and coated onto the surface of the first coating layer, and then dried at room temperature for 5 minutes; Finally, the polystyrene core was removed by calcination to obtain Ti ₓ - CPARCs.
Chemical structure analysis
Using a Brooke D8Focus X-ray diffractometer (XRD), crystal information was analyzed under conditions of 40kV and 40mA using copper K α radiation (λ=0.1542nm). Record Fourier transform infrared (FTIR) spectra in the wavenumber range of 400-4000cm ⁻¹ using a Bruker INVENIO spectrophotometer. Firstly, scrape off the anti reflective coating on BK7 from the glass and collect it. Measure the powder using attenuated total reflection (ATR) mode. Use Thermo Fisher Scientific ESCALAB250Xi for X-ray photoelectron spectroscopy (XPS) testing and argon gas for deep etching testing.
contact angle measurement
The water contact angles (WCAs) of the samples were measured using the DataphysicsOCA25 contact angle system at ambient temperature. Carefully drop 3 microliters of water droplets onto the coating surface to measure the water contact angle. To reduce errors, the measured water contact angle of the sample is the average of measurements taken in five different regions.
Self cleaning performance
The self-cleaning performance of Ti ₓ - CPARCs was tested through the following steps. Immerse the four prepared Ti ₓ - CPARCs vertically into 25mL methylene blue (MB, 5mg/L) solution, and use a 365nm ultraviolet light source (using a handheld 6W UV analyzer) to fix the distance between the sample and the light source at 10cm. Then, irradiate the sample with ultraviolet light for 30 minutes under dark conditions to eliminate the effects of self adsorption and self degradation.
Use a UV visible spectrophotometer to monitor the changes in MB solution concentration (changes in MB absorption peak) and evaluate the photocatalytic degradation effect of Ti ₓ - CPARCs on MB. In the comparative experiment of PT05, JWN-TO-A01, and Degussa P25TiO ₂, the catalyst concentration was 1mg/mL.
According to the following equation, use the apparent rate constant k (min ⁻¹) to evaluate the photocatalytic reaction rate:
image
Among them, C0 and C are the concentrations of methylene blue (MB) before and after illumination, k is the apparent rate constant (min ⁻¹), and t is the illumination time (min).
Wear resistance and weather resistance
Non woven fabric (NWF) abrasion resistance tests were conducted on CPARCs and Tix CPARCs. The process is defined as follows: Place a 500 gram non-woven fabric on the coating, move it 3 centimeters, and repeat 40 cycles. By comparing the changes in transmittance and photocatalytic performance of the coating before and after friction, the wear resistance of the coating can be determined. For outdoor weather resistance, place the sample in an undisturbed outdoor environment and measure the transmittance every month to record the changes in its optical performance, in order to evaluate the weather resistance of different samples. Five parallel samples were prepared to reduce errors.
Results and Discussion
Structure and surface morphology of colloidal particles and CPARCs
Figure 2 (a) shows the typical transmission electron microscopy (TEM) morphology of polystyrene latex particles. It can be seen that the polystyrene latex particles are circular and have excellent dispersibility. The graph in Figure 2 (a) represents the particle size distribution of latex particles, indicating that the average diameter calculated by Gaussian simulation method is approximately 64.8 ± 1.4 nanometers. In addition, according to the results of dynamic light scattering (DLS), its polydispersity index (PDI) is less than 0.1, indicating good dispersibility.
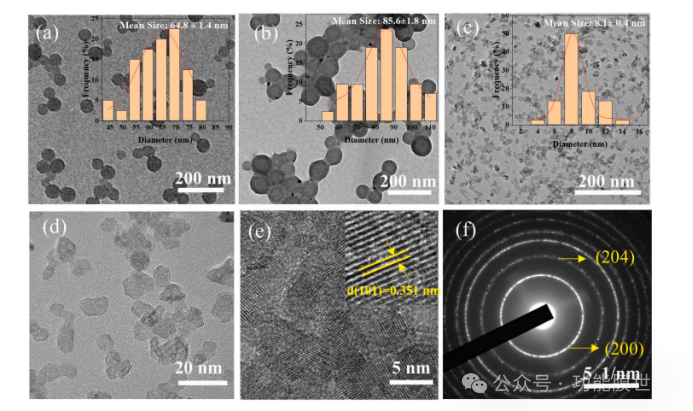
Figure 2. Transmission electron microscopy (TEM) images and particle size distribution of different samples: (a) Polystyrene template particles; (b) Core shell polymer/silica composite particles; (c) Titanium dioxide hydrosol; High resolution transmission electron microscopy (HRTEM) and selected area electron diffraction (SAED) images of PT05 nano titanium dioxide.
The structure and morphology of polystyrene/silica composite particles with core-shell structure are shown in Figure 2 (b). The results showed that silica can be well coated on polystyrene templates to form core-shell composite particles with a shell thickness of about 10 nanometers, a clean background, and almost no newly formed secondary particles, which provides a basis for the uniformity of coating formation.
As expected, the image in Figure 2 (b) confirms the formation of composite nanospheres with a size of approximately 85.6 ± 1.8 nanometers, ensuring that the pore size of closed cell silica is less than 100 nanometers to avoid capillary condensation [32]. The TEM image of the nano titanium dioxide dispersion is shown in Figure 2 (c). It can be seen that nano titanium dioxide has good dispersibility in water without obvious agglomeration phenomenon. The image in Figure 2 (c) shows that the average particle size of nano TiO ₂ estimated by Gaussian simulation is about 8.6 ± 0.4 nanometers, slightly larger than the initial particle size of 5 nanometers. In Figure 2 (e), lattice fringes (0.351 nanometers) of PT05 nano TiO ₂ were observed using high-resolution transmission electron microscopy (HRTEM), corresponding to the (101) crystal plane of rutile phase. In addition, the selected area electron diffraction (SAED) pattern of PT05 nano TiO ₂ in Figure 2 (f) shows distinct concentric rings corresponding to the (200) and (204) crystal planes of TiO ₂.
The cross-sectional and surface morphology of CPARCs and Ti1000 CPARCs are shown in Figures 3 (a) -3 (d). It can be observed that CPARCs are composed of silica nanospheres with diameters ranging from 60 to 100 nm. As shown in Figure 3 (a), these silica nanospheres adhere to each other, forming a continuous surface with a micro convex structure. Figure 3 (b) shows the corresponding cross-sectional morphology scanning electron microscopy images of CPARCs. It can be seen that the coating is composed of hollow nano silica.
The sphere exhibits a closed pore structure with a thickness of approximately 120 nanometers. The diameter of the nanopore structure is very close to that of the polystyrene template particles. The wall thickness of hollow silica spheres is about 10-20 nanometers. From Figure 3 (c), it can be observed that nano TiO ₂ is uniformly distributed on the surface of CPARCs, forming a structure similar to raspberry, and no agglomeration occurs after calcination. The particle size of nano TiO ₂ remains almost unchanged before and after calcination. This is beneficial for improving the optical performance and photocatalytic activity of Ti1000 CAPRCs. The side profile of Ti1000 CPARCs in Figure 3 (d) shows that the coating surface becomes smoother compared to CPARCs. The energy dispersive spectroscopy (EDS) mapping and EDS spectral results in Figures 3 (e-i) and 3 (f) further confirm that Ti, O, and Si atoms are uniformly distributed in Ti1000 CAPRCs.
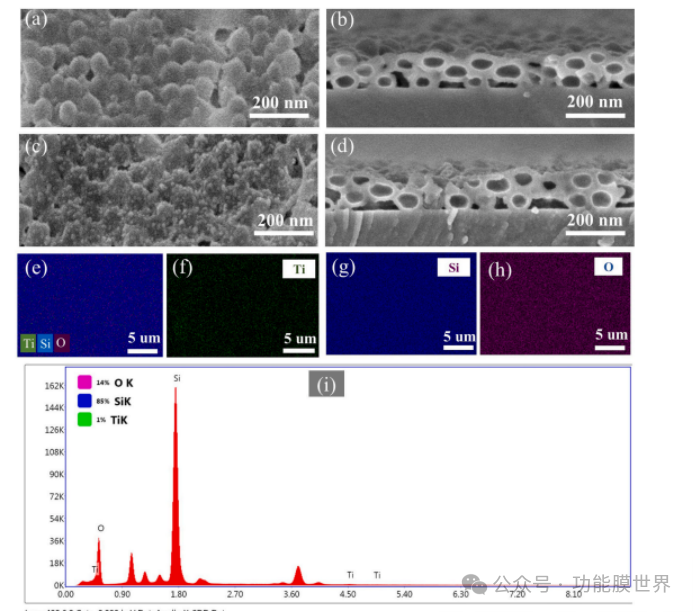
Figure 3. Scanning electron microscopy (SEM) images of CPARCs (a, b) and Ti1000 CPARCs (c, d); Corresponding Si, O, Ti surface element mapping maps (e-h) and energy dispersive spectroscopy (EDS) maps (i).
Chemical structure analysis
The crystal form and grain size of titanium dioxide can affect its photocatalytic and optical properties. Therefore, in order to confirm the existence and stability of rutile TiO ₂, X-ray diffraction (XRD) was used to analyze PT05 nano TiO ₂ and Ti1000 CPARCs before and after heat treatment, as shown in Figure 3 (a). It can be observed that PT05TiO ₂ before calcination exhibits typical diffraction peaks of rutile titanium dioxide at 2 θ=25.31 °, 37.88 °, and 48.06 °, corresponding to the (101), (004), and (200) crystal planes, respectively [13]. In addition, the characteristic peak shape of the (101) crystal plane of PT05TiO ₂ before calcination has become relatively sharp, indicating its high crystallinity. Compared with the original PT05TiO ₂, the diffraction peak positions of the PT05TiO ₂ samples after heat treatment at 550 ℃ and 650 ℃ remained almost unchanged, and no new diffraction peaks appeared. This indicates that under the current heat treatment conditions, the rutile phase did not transform into the rutile phase. In addition, it can be observed that after calcination at 650 ℃, the diffraction peaks of the sample become sharper and narrower, indicating more complete crystallization. The increase in crystallinity is beneficial for the photocatalytic ability of PT05TiO ₂. Therefore, 650 ℃ was chosen as the heat treatment temperature for Tix CPARCs.
As shown in Figure 4 (a), the XRD spectrum of Ti1000 CPARCs is similar to that of the original nano titanium dioxide. In addition, the grain size of nano TiO ₂ can affect the optical properties of the coating. Therefore, use the Scherrer formula to calculate the grain size of all samples. The calculation results indicate that the grain size of the original PT05TiO ₂ is 9.7nm, slightly larger than the data provided by the supplier. After heat treatment at 550 ℃ and 650 ℃, the crystal size increased to 21.8 nanometers and 27.5 nanometers, respectively. Surprisingly, the crystal size of nano TiO ₂ on the coating was only 11.7 nanometers and did not significantly increase. This is consistent with the results of scanning electron microscopy (SEM) images, indicating that TiO ₂ is well dispersed on the surface of CPARCs (it is unclear what CPARCs are here, depending on the context), which is a prerequisite for maintaining good photocatalytic performance.
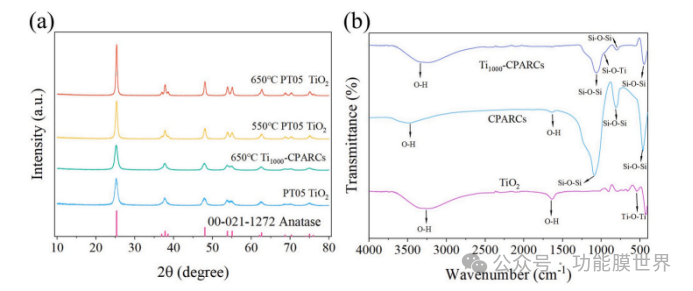
Figure 4. (a) X-ray diffraction (XRD) patterns before and after heat treatment (b) Fourier transform infrared spectra (FT-IR) of TiO ₂, Ti1000 CPARCs, and CPARCs
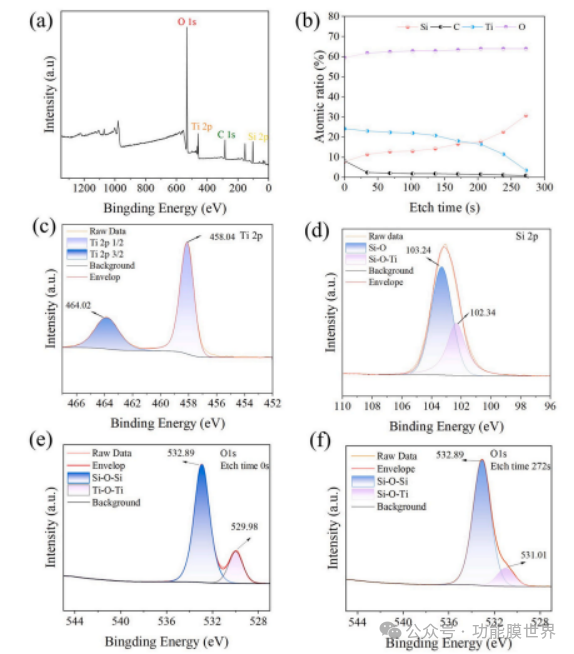
Figure 5. X-ray photoelectron spectroscopy (XPS) of Ti1000 CAPRCs: (a) full spectrum range, (b) variation of element ratio with etching time, (c) titanium (Ti) 2p orbitals, (d) silicon (Si) 2p orbitals, (e) oxygen (O) 1s orbitals at etching time of 0 seconds, (f) oxygen (O) 1s orbitals at etching time of 272 seconds.
Figure 4 (b) shows the Fourier transform infrared (FT-IR) spectra of TiO ₂, CPARCs, and Ti1000 CPARCs. For the TiO ₂ spectrum, the absorption band around 561cm ⁻¹ is caused by Ti-O bonds, and the peak at 1632cm ⁻¹ is attributed to the - OH group originating from molecular water. In CPARCs, the absorption bands near 1092cm ⁻¹, 802cm ⁻¹, and 461cm ⁻¹ belong to the asymmetric stretching vibration, symmetric stretching vibration, and bending vibration of Si-O-Si bonds, respectively. Compared with the spectra of CPARCs, Ti1000 CPARCs exhibit a new absorption peak at 958cm ⁻¹, which is attributed to the stretching vibration of Si-O-Ti bonds [35]. This result demonstrates the formation of chemical bonds between nano titanium dioxide and SiO ₂ on the surface of CPARCs after calcination. The broad absorption peak between 3000cm ⁻¹ and 3500cm ⁻¹ originates from the stretching vibration of the - OH group, proving the existence of H ₂ O adsorption on the TiO ₂ surface.
The XPS photoelectron spectra containing C, O, Si, and Ti elements are shown in Figure 5 (a). At the same time, deep etching analysis of Si and Ti elements was conducted on the coating, and the variation of element ratios over time was obtained through deep etching analysis, as shown in Figure 5 (b). As the etching time increases, the proportion of titanium element significantly decreases, while the proportion of silicon element gradually increases, which confirms that titanium dioxide mainly exists on the surface of the coating. Figure 5 (c) shows two peaks in the Ti2p spectrum, located at 458.04eV and 464.02eV, corresponding to Ti2p3/2 and Ti2p1/2, respectively. The Si2p spectra of the coating can be fitted to 102.34eV and 103.24eV in Figure 5 (d). O1s XPS spectra confirm the presence of Ti-O-Ti, Si-O-Ti, and Si-O-Si bonds, with binding energies of 529.98eV, 531.01eV, and 532.89eV, respectively [37,38]. It is worth noting that during the etching time of 0s-272s in Figure 5 (d and e), the 529.98eV binding energy absorption peak of Ti-O-Ti decreased, and the O1s XPS spectrum analysis showed that the 531.01eV binding energy absorption peak of Si-O-Ti appeared at the sixth etching level, indicating the formation of Si-O-Ti bonds at the interface between TiO2 and SiO2. The XPS and FT-IR spectra obtained are in good agreement, indicating the formation of Si-O-Ti bonds in the coating.
optical performance
Figure 6 (a) shows the changes in optical properties of composite anti reflective coatings (CPARCs) as the pulling rate increases from 700 μ m/s to 1500 μ m/s. Detailed data are listed in Table 1. It can be seen that as the pulling rate increases, the peak of optimal transmittance undergoes a red shift, indicating that the coating thickness gradually increases. When the pulling rate is 900 μ m/s, CPARCs exhibit the best optical performance. The highest transmittance of the coating at 548nm is 99.34%, and the average transmittance in the wavelength ranges of 400-700nm and 400-1100nm are 97.46% and 95.78%, respectively. Compared with the blank glass, the gain effect is 6.69% and 5.97%, respectively. Therefore, the first layer of CPARCs was prepared under the condition of a pulling rate of 900 μ m/s, and the content of nano TiO ₂ particles on the surface of CPARCs was controlled by adjusting the pulling rate. Figure 6 (b) shows the changes in the optical properties of the coating, with detailed data listed in Table 2. It can be observed that the transmittance of the coating has decreased compared to CPARCs. As the pulling rate increases, the content of nano TiO ₂ particles on the surface of CPARCs increases, and the transmittance of the coating decreases. Ti500 CPECs only decreased by 0.53% in the 400-700nm wavelength range, however, Ti2500 CPECs unexpectedly decreased by 4.72%, which is close to the transmittance of blank glass and almost loses its anti reflection ability. The transmittance of Ti1000 CPECs in the entire wavelength range can exceed that of blank glass, which is the maximum rate for maintaining high transmittance of glass. In order to eliminate the negative effects of nano TiO ₂, a double-layer anti reflection coating was designed based on the design theory of λ/4- λ/4 double-layer coatings.
Table 1 Transmittance of CPARCs at Different Shrinkage Rates.
Table 2 Transmittance of Ti ₓ - CPARCs at different pulling rates.

The optical layer design software TFCalc was used for simulation design, and the simulation results are shown in Figures 6 (c) and (d). When using an anti reflective coating (ACSS) with a refractive index of 1.45 as the bottom layer and a composite porous anti reflective coating (CPARCs) with a refractive index of 1.26 as the top layer. Through simulation, it is known that the thickness of the bottom and top layers of the coating is 95 nanometers and 109 nanometers, respectively. The coating thickness is adjusted by the pulling speed, and the relationship curve between coating thickness and pulling speed is shown in Figure S1. Under these conditions, simulation results show that the average transmittance of the coating can reach 98.89% in the wavelength range of 400-700 nanometers. However, the experimental results showed that the average transmittance of the double-layer composite porous anti reflective coating in the range of 400-700 nanometers was 98.54%. The actual transmittance is slightly lower than the simulation results, which may be due to inaccurate control of coating thickness or defects in the coating. However, it is satisfactory that the optical performance of the double-layer Ti1000 composite porous anti reflective coating has been improved, with 0.08% and 0.07% higher than that of the single-layer composite porous anti reflective coating in the range of 400-1100 nanometers and 400-700 nanometers, respectively. The transmittance data of the double-layer coating are listed in Table 3.
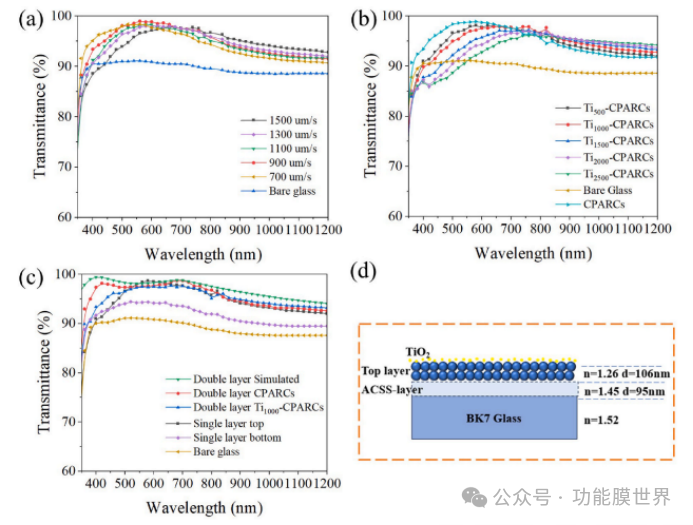
Figure 6. UV Vis NIR spectra of CPARCs (a), Tix CPARCs (b), and bilayer coating (c) at different pulling rates; Schematic diagram of double-layer coating structure (d).
Table 3 Transmittance of Double Layer Coating
photocatalytic performance
Degussa's P25 titanium dioxide (TiO ₂) powder is commonly used in the field of photocatalysis. Therefore, in this study, it was selected as a control sample for the comparison of photocatalytic ability. As shown in Figure 7, the photocatalytic ability of PT05 titanium dioxide and JWN-TO-A01 titanium dioxide is superior to that of Degussa's P25 titanium dioxide. The apparent rate constants k of PT05, JWN-TO-A01, and P25 titanium dioxide are 0.01699, 0.01257, and 0.00736, respectively. In addition, among these three samples, PT05 titanium dioxide powder has the best water dispersibility and stability. Therefore, it was selected as a photocatalytic modifier for coatings. After calculation, the content of titanium dioxide in Ti1000 CPARCs is only 2.38wt%. In order to investigate the difference in photocatalytic activity of titanium dioxide on the surface and inside of coatings, a titanium dioxide aqueous dispersion was directly mixed with a core-shell structured polystyrene/silica composite particle dispersion to prepare a mixed dispersion, ensuring a titanium dioxide content of 2.38wt%. By controlling the pulling speed, a mixed TiO ₂ - SiO ₂ coating was prepared, and its titanium dioxide content was kept consistent with Ti1000 CPARCs. As shown in Figures 8 (b) and (c), the degradation rate of methylene blue (MB) by Ti1000 CAPRCs sample is 76.2%, while the degradation rate of the reference sample is only 64.1%. The results indicate that titanium dioxide on the surface of the coating is more helpful for the degradation of methylene blue.
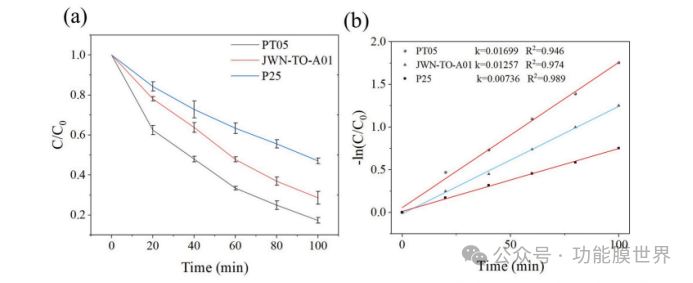
Figure 7. Photocatalytic degradation of PT05, JWN-TO-A01, and P25 under 365 nm ultraviolet light irradiation (a) and first-order kinetic fitting (b).
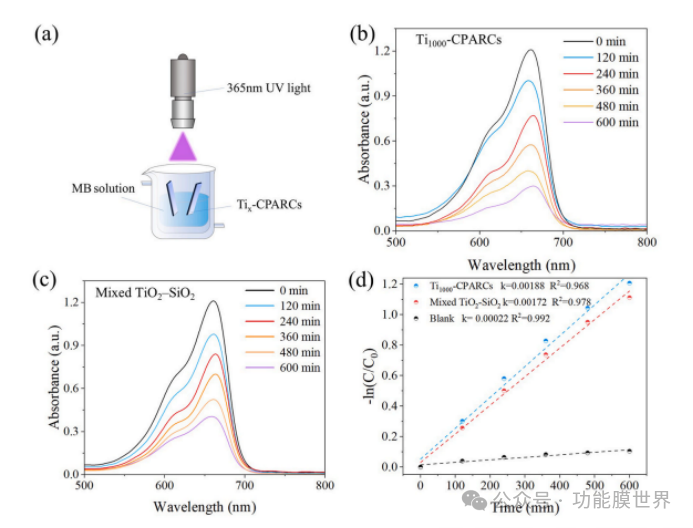
Figure 8.5mg/L Schematic diagram of photocatalytic degradation of methylene blue (MB) (a); UV visible absorption spectra of methylene blue solution catalyzed by Ti1000 CAPRCs (b) and reference coating catalyzed methylene blue solution (c); The relationship diagram between ln (C/C ₀) and reaction time (d).
Figure 8 (d) shows that the photocatalytic degradation of methylene blue (MB) in all samples follows a clear quasi first order kinetics. The apparent rate constants k of Ti1000 CPECs, TiO ₂ - SiO ₂ mixed samples, and blank samples are 0.00188, 0.00172, and 0.00022, respectively. The photocatalytic efficiency of the reference sample is lower than that of Ti1000 CAPRCs, indicating that directly mixing nano TiO ₂ into the system will lead to a decrease in the photocatalytic performance of the coating. The decrease in photocatalytic performance is mainly attributed to some nano TiO ₂ particles being buried inside the coating, which is ineffective for MB degradation. Therefore, in this system, TiO ₂ exhibits better photocatalytic performance on the surface.
wettability
From the two-dimensional images, line scan contours, and three-dimensional images in Figures 9 (a-c), it can be seen that the surface of CPARCs (specific reference is not clear here, context needs to be considered) has regular protrusions and depressions, similar to the scanning electron microscope (SEM) image of the sample. In contrast, after embedding TiO ₂, a raspberry like structure was formed on the surface of CPARCs. Therefore, in the atomic force microscopy (AFM) images of Ti1000 CPARCs, there were many smaller protrusions, resulting in a significant increase in roughness, as shown in Figure 9 (d-f). The root mean square (RMS) roughness values before and after coating with TiO ₂ were 3.8 nanometers and 11.1 nanometers, respectively.
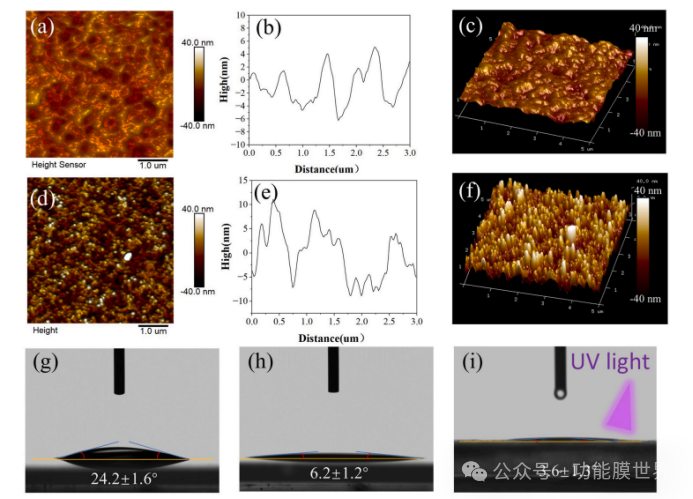
Figure 9: Atomic Force Microscopy (AFM) 2D images, line scan profiles, and 3D images of CPARCs (a-c) and Ti1000 CPARCs (d-f); Water contact angle (WCA) (g-i) of CPARCs, Ti1000 CPECs, and Ti1000 CPECs under ultraviolet light.
The nano roughness formed significantly affects the wettability of the coating surface. The contact angle of the coating decreased from 24.2 ° to 6.2 °. After 1 hour of exposure to ultraviolet light, the contact angle can be reduced to 3.6 ° and a superhydrophilic surface can be formed. This result indicates that the coating exhibits excellent superhydrophilic properties, thereby achieving a synergistic self-cleaning effect with the photocatalytic performance of TiO ₂.
Wear resistance and weather resistance
Wear resistance is one of the important properties in the practical application of anti reflective coatings (ARCs). The schematic diagram of the wear resistance strength test of the new wear-resistant material (NWF) coating is shown in Figure 10 (a). Figures 10 (b) and (c) show the optical performance changes of composite porous anti reflective coatings (CPARCs) and 1000 ℃ calcined titanium doped composite porous anti reflective coatings (Ti1000 CPARCs) before and after 40 wear cycles. The average transmittance of CPARCs slightly decreased from 97.01% to 96.74% within the wavelength range of 400-1100nm, ΔT=0.29%。 The transmittance of Ti1000 CPARCs in this wavelength range decreased from 97.11% to 96.34%, with a decrease of Δ T=0.77%, which is greater than the decrease of CPARCs. The possible reason is the partial detachment of TiO ₂ on the surface of the coating. Both samples meet the maximum attenuation value specified in the International Electrotechnical Commission (IEC) standard IEC51215, which is less than 1.00%.
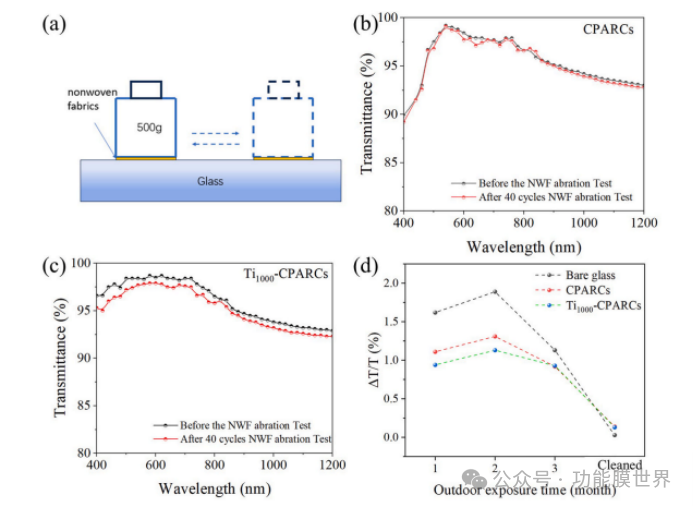
Figure 10. Schematic diagram of natural weathering friction (NWF) wear test (a); UV visible near-infrared spectra of CPARCs (b) and Ti1000 CPARCs (c) after 40 wear cycles; Outdoor exposure experiment (d).
Figure 10 (d) shows the optical performance changes of blank glass, CPARCs, and Ti1000 CPARCs after 3 months of outdoor exposure experiments. The pollution situation is defined by the ratio of the average transmittance decrease Δ T in the wavelength range of 400-1100nm to the average transmittance T. All samples' surfaces were contaminated to varying degrees, with blank glass being the most severely contaminated. CPARCs and Ti1000 CPARCs showed significant improvement, which is related to the good hydrophilicity of the coating surface. The fluctuation of values in different months may be related to the rainfall situation of that month. After cleaning, surface pollutants were effectively removed and there was no significant damage to the sample surface, indicating that CPARCs and Ti1000 CPARCs have good weather resistance and are expected to achieve practical applications.
Conclusion
Successfully prepared TiO ₂ modified anti reflective coatings (ARCs) with excellent optical properties and super hydrophilic photocatalytic self-cleaning function.
By using several nanometers of rutile TiO ₂ to form a raspberry like structure on the surface of the coating and designing a double-layer coating, Ti ₁₀₀₀ - closed cell anti reflection coatings (CPARCs) have better optical properties than ordinary closed cell anti reflection coatings. This design perfectly solves the contradiction between optical performance and photocatalytic performance.
Compared with other studies in Table S1, this method enables nano TiO ₂ to be modified on the surface of closed cell coatings, fully exerting its photocatalytic effect and significantly reducing the amount of TiO ₂ used, which is beneficial for improving efficiency and reducing costs. This is currently the situation where TiO ₂ is used the least amount in TiO ₂ modified silica anti reflective coatings. In addition, research has found that through the calcination process, Si-O-Ti chemical bonds are formed between nano titanium dioxide and silicon dioxide on the surface of the closed cell anti reflection coating, improving the adhesion between titanium dioxide and the coating and providing strong support for the durability and wear resistance of the coating. Ti ₁₀₀₀₀ - closed cell anti reflection coating has excellent wear resistance and weather resistance. This is a novel and effective method for preparing high-performance multifunctional TiO ₂ - modified silica based anti reflective coatings.
Welcome to Changzhou Hefeng Packaging Materials Co., Ltd!
Mobile phone: 15895061333
Email: 115555372@qq.com
Address: Moujia Village, Zhenglu Town, Tianning District, Changzhou City
Copyright © 2025 Changzhou Hefeng Packaging Materials Co., Ltd